Rifaximin is an antibiotic that does not enter the body. Rifaximin is used in the treatment of traveler’s diarrhea (which is also the use of Mutaflor, Myrisan and other probiotics) and would have significant potential with CFS. While there has been no PubMed studies with CFS, there has been some for IBS which I view as a different manifestation of the same condition.
- “Although rifaximin had significant improvement in symptoms of IBS over placebo, it is notable that only 40.7% patients had a response to treatment with a small incremental benefit compared with 31.7% improvement in placebo group.[2] Treatment for IBS with rifaximin should be prudent.” [2014]
- “Clinical studies have demonstrated that rifaximin improves symptoms associated with IBS, such as bloating, flatulence, stool consistency, and abdominal pain, and has a side-effect profile similar to placebo..additional investigation into optimal dosing, treatment duration, and potential resistance is required”[2015]
The above article summaries results. It results in improvement and not remission.
| Dosage | Result |
|---|---|
| 400 mg bid for 10 days | Higher global improvement in IBS symptoms with rifaximin (41.3% vs 22.9%, P=0.03). Lower mean symptoms score and bloating with rifaximin |
| 400 mg qid for 14 days | Higher global improvement in IBS symptoms with rifaximin (36.4% vs 21%, P=0.02). Bloating improved with rifaximin |
| 200 mg qid for 14 days | Improved overall well-being (3.9% vs 2.7%, P<0.001), bloating (5.5% vs 3.6%, |
| 550 mg qid for 14 days | Higher global improvement in IBS symptoms with rifaximin (40.8% vs 31.2%), Rifaximin group had more relief of bloating (39.5% vs 28.7%) |
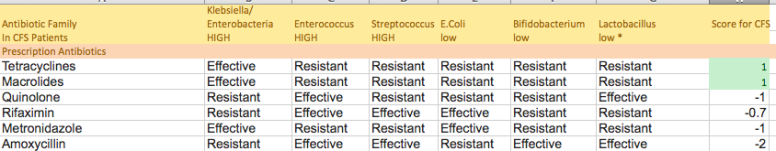
The model that I use for CFS/FM/IBS is overgrowth of (Klebsiella, Enterobacteria, Enterococcus,Streptococcus) and very low levels of (E.Coli, Bifidobacterium, Lactobacillus) reported first in 1998. Looking at the profile reported in a 2013 article, rifaximin inhibited in vitro:
- 85.4% of Escherichia coli (BAD)
- 43.6% of Klebsiellaspp., (Good – but too little)
- 34.8% of Enterobacter spp (Good – but too little)
- 54.5% of other Enterobacteriaceae spp., (Good – but too little)
A 2014 study reported
- Active against 96.9% diverse Enterobacteriaceae and
- 90% of Campylobacter spp. were resistant
This appears consistent with the results reported above– some improvement would be expected but the core shifts would not be corrected.
Checking the general web, I find in this post:
“Usage amongst ME/CFS Specialists
Dr. De Meirleir uses Rifaximin on patients based on test results, including this dysbiosis test. He sometimes combines it with other antibiotics depending on test results and recommends the Rifaximin be followed by a 23 day course of the probiotic VSL#3
Dr. Teitelbaum believes that all ME/CFS patients be at least tested for SIBO. He writes about his theories of SIBO here.
Dr. Peterson seems to prescribe Rifaximin to a number of his patients with some taking the probiotic VSL#3 after the Rifaximin course.
Dr. Myhill recommends Rifaximin to some patients. Although she has a different dosing strategy to most, involving 200mg 3x a day for 3 days followed by a maintenance dose of 200mg daily. She also incorporates a hydrogen sulphide urine test to monitor progress. She elaborates on this here.”
Benefit/Risk Odds
For rifaximin, “The overall eradication rate according to intention-to-treat analysis was 70.8%…The overall rate of adverse events was 4.6%… improvement or resolution of symptoms in patients with eradicated SIBO was found to be 67.7%”[2017]
- “Although the rifaximin group showed a greater percentage of global symptom improvement, this was limited to bloating, as scores for abdominal pain, diarrhea, and constipation did not improve significantly.”[2007]
Bottom Line
In my ranking of antibiotics, it is reasonable but still not ideal as shown below. It is not part of a cure but looks like a reasonable compliment to tetracyclines and macrolides to be given concurrently.