This post extends the analysis of microbial involvement in ME/CFS pathophysiology by focusing on metabolites produced or consumed by bacteria, rather than on individual bacterial species seen in the earlier post Microbial involvement in myalgic encephalomyelitis/chronic fatigue syndrome pathophysiology. . This shift in perspective is valuable because:
Metabolite-Centric Analysis
Bacterial Metabolic Activity: Bacteria produce and consume various metabolites, which can significantly impact the host’s metabolic environment13.Metabolic Imbalances: Different bacterial compositions can lead to similar metabolite imbalances, making metabolite profiles potentially more informative than bacterial species profiles alone7 8.
Advantages of This Approach
- Net Effect: By examining metabolites, we can assess the overall impact of the microbiome on the host, regardless of the specific bacterial species present5.
- Consistency: Metabolite imbalances may be more consistent across patients than bacterial species composition, which can vary widely7.
- Functional Insight: This approach provides insight into the functional consequences of microbiome dysbiosis in ME/CFS3 8.
KEGG Application
Using the KEGG: Kyoto Encyclopedia of Genes and Genomes,(KEGG) allows for:
- Mapping of metabolites to specific pathways
- Identification of key metabolic alterations in ME/CFS patients
- Potential discovery of new biomarkers or therapeutic targets7
Metabolite Profiling in ME/CFS
Recent studies have identified several metabolic alterations in ME/CFS patients:
- Disruptions in energy metabolism and mitochondrial function2 5
- Alterations in lipid metabolism, including changes in ceramides and complex lipids4
- Disturbances in amino acid metabolism8
Clinical Implications
Understanding metabolite profiles in ME/CFS could lead to:
- Improved diagnostic tools
- Identification of potential therapeutic targets
- Personalized treatment approaches based on individual metabolic profiles58
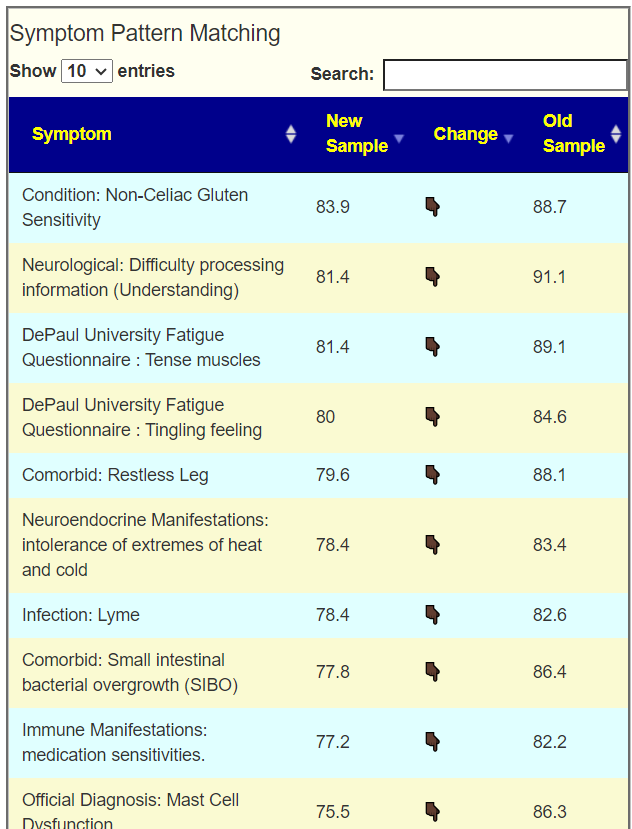
I am showing the numbers for Biomesight sample below. Conclusions across Ombre, uBiome and Biomesight are at the bottom.
Warning: These are the chemical names — a few are available as supplements with more common name.
BiomeSight Results
I did three slice-and-dice
- Producers
- Consumers
- Net metabolites (Producers – Consumers) – this is like the most important
Remember: results may be different for different labs. Also, these are estimates of the metabolites
Metabolite Producers
- DNA N4-methylcytosine <= 31.1
- Cytidine 5′-diphosphoramidate <= 28
- Pyridoxal <= 24.3
- Allantoate <= 23.6
- [L-Glutamate:ammonia ligase (ADP-forming)] <= 22.9
- Adenylyl-[L-glutamate:ammonia ligase (ADP-forming)] <= 22.9
- Uridylyl-[protein-PII] <= 22.6
- 4-Hydroxybenzoate <= 19.6
- 5-Phospho-D-xylonate <= 18.5
- 5-Phospho-L-arabinate <= 18.5
- Formyl-CoA <= 18
- Aminoacrylate <= 17.3
- Methylaminoacrylate <= 17.3
- Acetoacetate <= 17.3
- 5-Carboxyamino-1-(5-phospho-D-ribosyl)imidazole <= 17.3
- UDP-N-acetyl-alpha-D-muramoyl-L-alanyl-L-glutamate <= 17
- N-Acyl-L-homoserine <= 16.7
- (R)-Piperazine-2-carboxylate <= 16.1
- 2-[(2-Aminoethylcarbamoyl)methyl]-2-hydroxybutanedioate <= 16
- D-Mannitol 1-phosphate <= 16
- D-Erythritol 1-phosphate <= 15.6
- 2-Acetylphloroglucinol <= 15.5
- 3-Dehydrocarnitine <= 15.2
- Deoxynucleoside <= 14.9
- Formaldehyde <= 14.5
- (S)-3-Acetyloctanal <= 14.4
- Pyrrole-2-carbonyl-[pcp] <= 14.4
- (L-Prolyl)adenylate <= 14.4
- (L-Arginyl)adenylate <= 14.4
- 2”-Nucleotidylgentamicin <= 13.9
- 4-O-(beta-L-Arabinofuranosyl)-(2S,4S)-4-hydroxyproline <= 13.3
- beta-L-Arabinofuranosyl-(1->2)-beta-L-arabinofuranose <= 13.3
- Polysulfide <= 13.2
- 6-Deoxy-6-sulfo-D-fructose <= 13
- 1-Phosphatidyl-1D-myo-inositol 5-phosphate <= 12.9
- 2-Dehydro-3-deoxy-D-galactonate <= 12.8
- beta-L-Arabinofuranose <= 12.8
- Maltose 6′-phosphate <= 12.7
- Cytidine <= 12.5
- [beta-GlcNAc-(1->4)-Mur2Ac(oyl-L-Ala-gamma-D-Glu-6-N-(beta-D-Asp)-L-Lys-D-Ala-D-Ala)]n <= 12.5
- O-Phospho-L-homoserine <= 12.3
- 2,5-Diamino-6-(5-phospho-D-ribosylamino)pyrimidin-4(3H)-one <= 12.3
- Butanoyl-CoA <= 12.2
- 4-Amino-5-hydroxymethyl-2-methylpyrimidine <= 12.1
- Oxalyl-CoA <= 12.1
- 5-(2-Hydroxyethyl)-4-methylthiazole <= 11.8
- 2-Hydroxyornithine lipid <= 11.5
- N3-Acetyl-2-deoxystreptamine antibiotic <= 11.3
- Protein histidine <= 11.3
- Protein N6-acetyl-L-lysine <= 11.2
- Protoporphyrinogen IX <= 11
- Divinylprotochlorophyllide <= 10.9
Metabolite Consumers (Substrates)
We have a shorter list with 5 metabolites bubbling to the surface as excessive metabolites.
- Linalool >= 96.4
- 6-Oxocyclohex-1-ene-1-carbonyl-CoA >= 96.4
- 2-epi-5-epi-Valiolone >= 96.2
- Lupanine >= 95.2
- 4′-Hydroxyacetophenone >= 95.2
- DNA cytosine <= 31.1
- Xylitol <= 29.4
- N5-(Cytidine 5′-diphosphoramidyl)-L-glutamine <= 28
- 6-Hydroxynicotinate <= 27.6
- Pyridoxine <= 24.1
- [L-Glutamate:ammonia ligase (ADP-forming)] <= 22.9
- Adenylyl-[L-glutamate:ammonia ligase (ADP-forming)] <= 22.9
- 2-Amino-2-deoxyisochorismate <= 22.7
- [Protein-PII] <= 22.6
- Formyl-CoA <= 20.4
- D-Erythrulose 4-phosphate <= 20 alpha-Maltose 1-phosphate <= 18.8
- L-Arabino-1,4-lactone 5-phosphate <= 18.5
- D-Xylono-1,4-lactone 5-phosphate <= 18.5
- N-Acyl-L-homoserine lactone <= 18.1
- Oxalyl-CoA <= 18
- Methylureidoacrylate <= 17.3
- Ureidoacrylate <= 17.3
- UDP-N-acetyl-alpha-D-muramoyl-L-alanyl-L-glutamate <= 17
- D-arabino-Hex-3-ulose 6-phosphate <= 17
- beta-Alaninamide <= 16.1
- (R)-Piperazine-2-carboxamide <= 16.1
- 2-[(L-Alanin-3-ylcarbamoyl)methyl]-2-hydroxybutanedioate <= 16
- Erythritol <= 15.6
- alpha-L-Rhamnopyranosyl-(1->3)-N-acetyl-alpha-D-glucosaminyl-diphospho-trans,octacis-decaprenol <= 15.5 2,4-Diacetylphloroglucinol <= 15.5
- (S)-Allantoin <= 15.1
- L-Prolyl-[pcp] <= 14.4
- trans-2-Octenal <= 14.4
- (L-Prolyl)adenylate <= 14.4
- (L-Arginyl)adenylate <= 14.4
- (5-L-Glutamyl)-L-amino acid <= 13.6
- 4-O-(beta-L-Arabinofuranosyl-(1->2)-beta-L-arabinofuranosyl-(1->2)-beta-L-arabinofuranosyl)-(2S,4S)-4-hydroxyproline <= 13.3
- Sulfoquinovose <= 13
- 1-Phosphatidyl-D-myo-inositol 4,5-bisphosphate <= 12.9
- D-Galactonate <= 12.8
- beta-L-Arabinofuranosyl-(1->2)-beta-L-arabinofuranose <= 12.8
- Oxalate <= 12.7
- N4-Acetylcytidine <= 12.5
- D-Aspartate <= 12.5
- [beta-GlcNAc-(1->4)-Mur2Ac(oyl-L-Ala-gamma-D-Glu-L-Lys-D-Ala-D-Ala)]n <= 12.5
- 3′-Phosphoadenylyl sulfate <= 12.4
- 2,5-Diamino-6-(5-phospho-D-ribitylamino)pyrimidin-4(3H)-one <= 12.3
- D-Xylulose 5-phosphate <= 12.2
- 4-Amino-5-aminomethyl-2-methylpyrimidine <= 12.1
- Deoxynucleoside 5′-phosphate <= 11.9
- Thiosulfate <= 11.6
- Ornithine lipid <= 11.5
- 2-Deoxystreptamine antibiotic <= 11.3
- UDP-alpha-D-galactofuranose <= 11.2
- 3′,5′-Cyclic AMP <= 11.1
- [Sulfatase]-L-serine <= 11
- trans-2,3-Dehydroacyl-CoA <= 10.9
- D-Serine <= 10.6
- 5,6-Dihydrothymine <= 10.4
- Electron-transferring flavoprotein <= 10.3
- D-Mannose <= 10.1
- Ethanol <= 10.1
Net Modifiers
Here we have shorter list with
- (R)-3-(4-Hydroxyphenyl)lactoyl-CoA >= 98.9
- 14alpha-Formylsteroid >= 98.7
- (E)-2-Methylgeranyl diphosphate >= 98.3
- Harderoheme III >= 97
- D-Erythritol 1-phosphate >= 83.4
- 1-(5-O-Phospho-beta-D-ribofuranosyl)-5-(sulfanylcarbonyl)pyridin-1-ium-3-carbonyl adenylate >= 56.8
- 8-Oxo-GDP <= 32.5 8-Oxo-dGDP <= 32.5
- 2,4-Diketo-3-deoxy-L-fuconate <= 27.2
- S-(Hercyn-2-yl)-L-cysteine S-oxide <= 27
- L-Formylkynurenine <= 24.6
- 2-[(2-Aminoethylcarbamoyl)methyl]-2-hydroxybutanedioate <= 23.5
- alpha-Oxo-benzeneacetic acid <= 23.4
- trans-o-Hydroxybenzylidenepyruvate >= 22.8
- 2-(alpha-D-Mannosyl)-3-phosphoglycerate <= 22
- Reduced FMN <= 20.6
- Deamino-NAD+ <= 19.7
- 5-(5-Phospho-D-ribosylaminoformimino)-1-(5-phosphoribosyl)-imidazole-4-carboxamide <= 18.5
- cis-2,3-Dihydroxy-2,3-dihydro-p-cumate >= 18.2
- Phthalate <= 17.9
- alpha-Ribazole <= 17.7
- beta-D-Fructose 6-phosphate <= 17.5
- Reduced electron-transferring flavoprotein <= 16.5
- GDP-L-fucose <= 16.4
- 3-Hydroxy-5,9,17-trioxo-4,5:9,10-disecoandrosta-1(10),2-dien-4-oate <= 15.4
- 2-Keto-D-gluconic acid <= 14.6
- 6-(Hydroxymethyl)-7,8-dihydropterin <= 14.4
- Formaldehyde <= 13.9
- Adenosyl cobyrinate hexaamide <= 13.6
- 3-Deoxy-D-manno-octulosonate <= 12.9
- L-Fuculose 1-phosphate <= 12.8
- D-Glutamate <= 12.5
- L-Tyrosyl-tRNA(Tyr) <= 12.1
- Maltose 6′-phosphate <= 11.8
- O-Phospho-L-serine <= 11.8
- 4-Guanidinobutanal <= 11.7
- 7-Carboxy-7-carbaguanine <= 11.7
- CDP-diacylglycerol <= 11.1
- Protoporphyrinogen IX <= 11.1
- 5-Guanidino-2-oxopentanoate <= 11
- N5-Phospho-L-glutamine <= 10.9
- D-1-Aminopropan-2-ol O-phosphate <= 10.5
- Thymine <= 10.5
- (2-Amino-1-hydroxyethyl)phosphonate <= 10.5
- Hydrogenobyrinate a,c diamide <= 10.2
- 2-Amino-3-carboxymuconate semialdehyde <= 10
Across Labs Consolidation
The analysis of metabolites across multiple microbiome testing platforms (Ombre, Biomesight, and uBiome) reveals a more consistent pattern of metabolite imbalances compared to bacterial species identification.
Potential Consequences of Low GDP-L-fucose (the top one)
The deficiency in GDP-L-fucose could have several implications:
- Altered Immune Response: It may affect the proper functioning of the immune system, potentially impacting inflammatory processes12.
- Cancer-Related Changes: Low levels might influence tumor progression or immune evasion mechanisms, as fucosylation is often altered in cancer24.
- First-degree relatives: A clinic-based study reported that first-degree relatives of ME/CFS patients had a significantly higher(four times) prevalence of any cancer compared to controls (OR 4.06) [2022]
- Cellular Communication: It could disrupt normal cell-cell interactions and signaling pathways dependent on fucosylated glycans3.
| Metabolite | Threshold | Low | High |
| GDP-L-fucose | 16.5 | 12.1 | 21.2 |
| Holo-[citrate (pro-3S)-lyase] | 12.7 | 1.8 | 23.4 |
| N,N’-Diacetyllegionaminate | 12.1 | 5.4 | 22 |
| alpha-Oxo-benzeneacetic acid | 11.8 | 1.9 | 23.4 |
| Oxalyl-CoA | 11.3 | 2 | 22.4 |
| S-Methyl-5-thio-D-ribose 1-phosphate | 11.2 | 1.3 | 30.6 |
| Malonyl-CoA | 10.6 | 2.5 | 26.3 |
| 1,2-Diacyl-3-alpha-D-glucosyl-sn-glycerol | 10.5 | 5.4 | 20 |
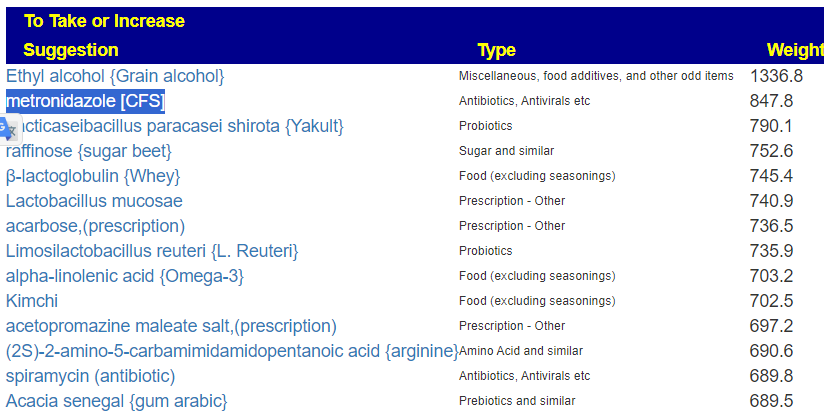
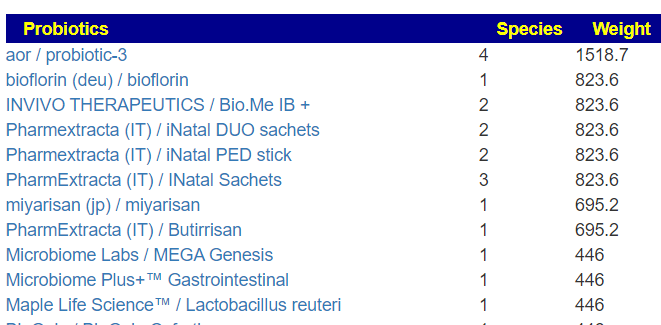
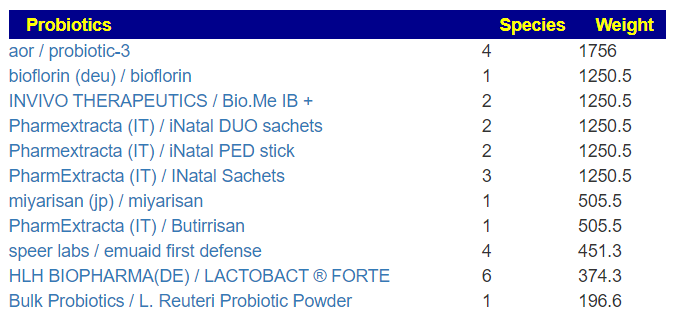
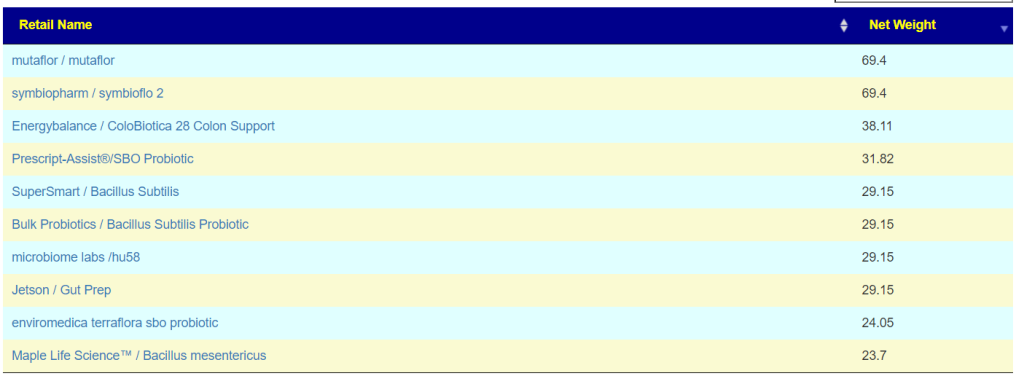
Translate Low Metabolites to Probiotics
Many of these metabolites are produced by probiotics, so in terms of highest importance and reasonably available probiotics, I produced the list below.
The top one is one is one that had very dramatic positive effect for me when I relapsed into ME/CFS (after the worse herxheimer reaction that I have ever experienced): Mutaflor (E.Coli Nissle 1917). I took it as a result of a 1999 study in Australia reporting very low levels of E.Coli in CFS patients [As a FYI, 16s tests do a very poor reporting on E.Coli].
The retail product microbiome labs/ megasporebiotic has several of the next on the list.
- Escherichia coli (Mutaflor, SymbioFlor-2) : 100%
- Bacillus thuringiensis: 70%
- Bacillus licheniformis: 67%
- Bacillus subtilis: 66%
- Bacillus subtilis subsp. natto: 67%
- Clostridium butyricum: 57% of the top
- Heyndrickxia coagulans (a.k.a. Bacillus coagulans): 65%
- Lactiplantibacillus plantarum: 59%
- Enterococcus faecalis: 57%
- Akkermansia muciniphila: 54%
I asked perplexity, which foods may increase any of the above metabolites. The following were reported:
- Spinach
- Rhubarb
- Beets
- Nuts
- Chocolate
- Tea
- Wheat bran
- Strawberries
Bottom Line
While the metabolite-focused approach provides valuable insights into the biochemical imbalances associated with ME/CFS, its immediate clinical applications are somewhat limited. The probiotics and the food suggestions are reasonable and I see several of the items appearing on suggestions from the expert system for ME/CFS patients.